
Meet us at Bengaluru Tech Summit in Hall No. - 1, Booth no. - C58
.png)
Agentic AI is beginning to change how early-stage drug development really works by taking on the documentation burden that quietly slows innovation.
In the U.S. biopharma ecosystem, the stakes couldn’t be higher. Bringing a new therapy from discovery to market often takes 10-15 years and can cost $2-3 billion per drug. At the same time, manufacturers are facing rising production costs, aggressive generic competition, and one of the most significant patent cliffs the industry has ever seen.
So the question is no longer whether innovation is needed, but where it can make the fastest and safest impact.
However, for many organizations, the bottleneck isn’t science. It’s documentation.
From clinical trial protocols and investigator brochures to quality records and FDA submissions, biopharma teams spend up to 30-40% of their time on documentation and compliance-related tasks. These processes are critical, no doubt! But they are also manual, repetitive, and slow, often delaying key milestones in development.
This is where Agentic AI in Biopharma is stepping in…
By intelligently managing high-volume, rules-driven workflows, Agentic AI is helping U.S. life sciences teams reduce documentation cycle times, improve accuracy, and free scientists to focus on what truly matters: innovation and patient outcomes. Importantly, this shift doesn’t compromise regulatory rigor. Instead, it strengthens compliance while boosting productivity.
As the pressure to deliver faster, safer, and more cost-effective therapies intensifies, can biopharma afford to rely on legacy operating models? Or is Agentic AI becoming a foundational capability for the next era of drug development?
The potential of Agentic AI reaches far beyond medical writing alone. In fact, generative AI is expected to scale faster in health care than in any other industry. Recent industry analysis indicates that the U.S. health care GenAI market is on track to expand at an extraordinary pace, growing from roughly $1 billion today to more than $20 billion by 2027, representing an annual growth rate of around 85%.
Together, these outcomes signal a clear shift that Agentic AI is becoming a strategic lever for speed, scale, and sustainable growth.
Most biopharma organizations are already familiar with AI and generative AI. Predictive models support trial design, and GenAI tools help draft content, summarize documents, or answer questions. But Agentic AI represents a structural shift.
The difference lies in autonomy, orchestration, and accountability.
Traditional AI and GenAI systems are largely reactive. They respond to inputs, execute predefined tasks, and stop. Agentic AI systems, by contrast, are goal-driven. They can plan, reason, act, evaluate outcomes, and iterate, often across multiple systems, while operating within defined guardrails.
Traditional AI behaves like a specialized instrument: highly effective at a single task but dependent on humans to decide what to do and when. GenAI improves productivity by generating language, code, or insights, but it still requires constant prompting and supervision.
Agentic AI functions more like a digital operator.
Once assigned an objective, such as preparing a clinical study report or managing a regulatory submission, an agentic system can:
This shift from task execution to autonomous workflow ownership is what makes Agentic AI especially powerful in complex, regulated environments like U.S. biopharma.
In FDA-regulated processes, accuracy, traceability, and consistency are non-negotiable. GenAI tools are helpful for drafting, but they lack context awareness, process memory, and governance intelligence.
Agentic AI systems are designed to operate within:
This allows organizations to scale automation without increasing regulatory risk.
For CXOs, the implication is clear that Agentic AI is not about replacing expertise. It is about codifying institutional knowledge into systems that execute reliably, at scale, and with full transparency.
GenAI improves how work is done, but agentic AI changes who owns the work, humans or systems.
For biopharma leaders navigating compressed timelines, talent constraints, and regulatory intensity, Agentic AI offers a path to:
The organizations that treat Agentic AI as a core capability, rather than a tool, will define the next operating standard for life sciences.
Agentic AI operates through a goal-driven lifecycle, rather than isolated prompts.
A human sets a high-level goal, such as:
“Prepare a Phase II clinical study report aligned with FDA submission standards.”
Unlike GenAI, the system is not told how to do it, only what success looks like.
The agentic system breaks the objective into structured tasks:
Dependencies and sequencing are determined automatically.
Specialized agents execute tasks in parallel:
Each agent operates within its defined scope, reducing error and rework.
Throughout execution, validation agents:
This replaces late-stage quality checks with built-in quality by design.
.png)
Humans remain in control:
The difference is that humans focus on judgment, not manual assembly.
Post-execution, the system:
Over time, the system becomes faster, more accurate, and more aligned with organizational standards.
Agentic AI aligns naturally with biopharma’s realities:
For CXOs, the value is not just efficiency but operational resilience.
Agentic AI enables organizations to:
In a market where speed, trust, and execution quality define winners, Agentic AI becomes an operating advantage.
For U.S. pharmaceutical companies, the case for Agentic AI is structural. The operating environment is becoming more constrained just as expectations for speed, safety, and transparency continue to rise. Regulatory scrutiny from the FDA, intensifying competition from generics and biosimilars, workforce shortages in regulatory and clinical functions, and increasing pressure on pricing and margins are forcing leaders to rethink how work gets done.
Agentic AI is emerging as a practical response to these pressures, driving three shifts that are particularly relevant in the U.S. pharma context.
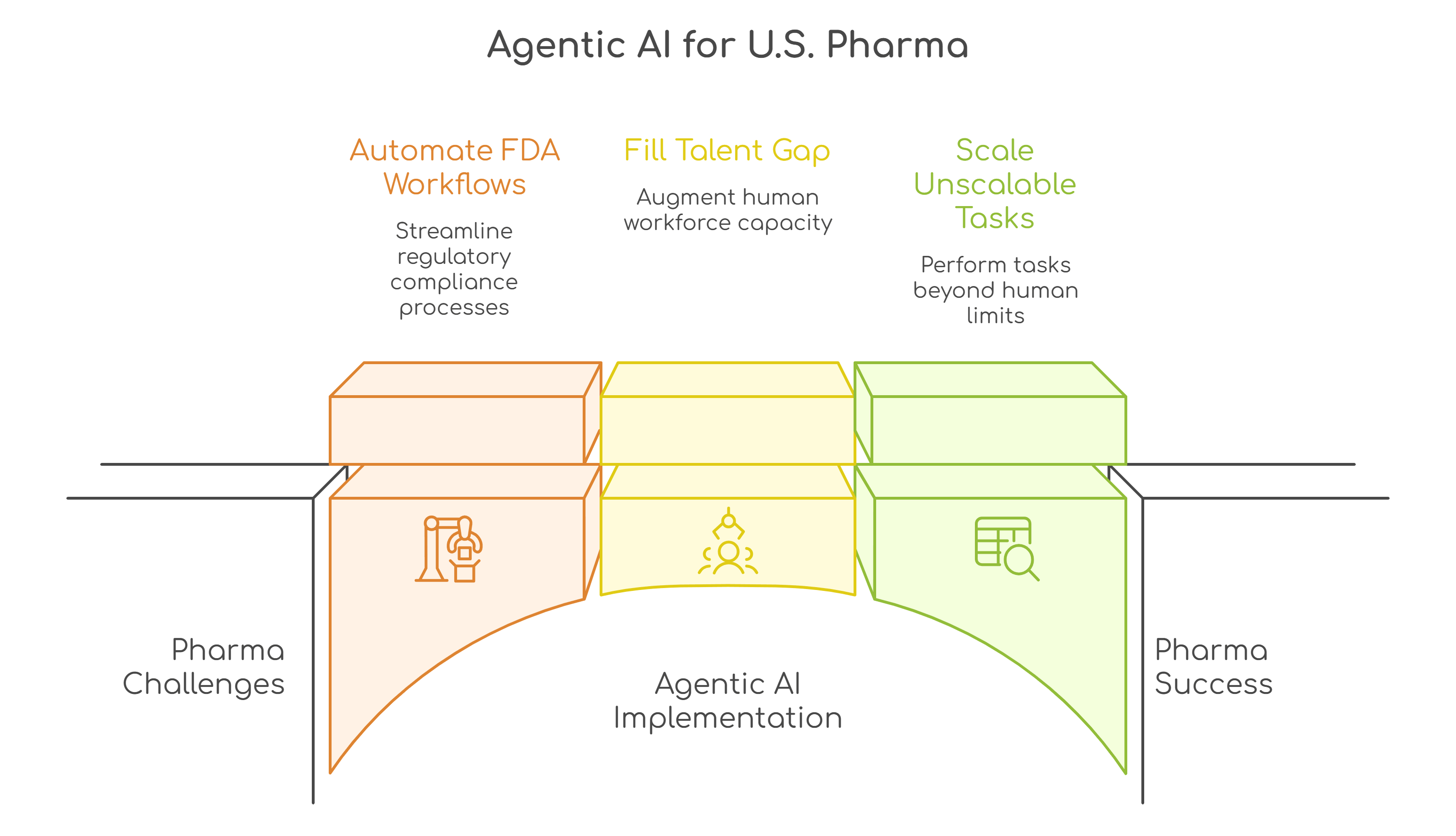
U.S. pharma companies operate in one of the most documentation-heavy regulatory environments in the world. From INDs and NDAs to post-market surveillance and safety reporting, a significant share of work is structured, repeatable, and rules-driven, yet still requires high accuracy and traceability.
Agentic AI can operate effectively within these constraints.
Most U.S. pharma workflows contain tasks that can be automated or augmented by agents without compromising regulatory rigor. Standardized activities, such as document assembly, cross-referencing, version management, and compliance checks, can be handled by lower-complexity agents configured by business teams. More advanced, FDA-facing workflows benefit from custom-built agents trained on internal SOPs, regulatory precedents, and submission history.
This allows organizations to scale compliance capacity without proportionally increasing headcount, a critical advantage given the shortage of experienced regulatory talent in the U.S. market.
U.S. pharmaceutical companies are facing a dual challenge: rising demand for specialized expertise and limited availability of experienced professionals in areas such as regulatory affairs, medical writing, pharmacovigilance, and quality assurance.
Agentic AI helps close this gap.
Rather than replacing expertise, agents act as force multipliers, taking ownership of execution-heavy tasks while humans focus on scientific judgment, regulatory interpretation, and decision-making. In practice, this means most U.S. pharma roles will increasingly work alongside agentic systems, configuring them, supervising outputs, and intervening when needed.
Over time, this model can release 25 to 40% of organizational capacity, enabling companies to:
For HR and leadership teams, this creates an opportunity to rethink job design, career paths, and operating models in a way that aligns with long-term workforce realities in the U.S.
Some of the most valuable work in U.S. pharma today is either underdone or not done at all, not because it lacks importance, but because it is too complex or resource-intensive for human teams to handle consistently.
Agentic AI changes this equation.
Agents can continuously analyze unstructured clinical data, scientific literature, safety reports, and real-world evidence to surface patterns that would otherwise go unnoticed. They can monitor long-tail operational and financial data to identify inefficiencies or compliance risks. They can also track brand and stakeholder sentiment across channels to provide always-on insight into market perception.
These capabilities are particularly valuable in the U.S., where scale, regulatory exposure, and market complexity amplify the cost of missed signals.
For U.S. pharmaceutical companies, Agentic AI directly impacts the metrics that matter most to leadership teams.
By accelerating development timelines, improving regulatory throughput, and increasing operational efficiency, agents can drive:
Roughly half of the value comes from top-line acceleration, better assets, faster approvals, and broader patient reach. The other half comes from efficiency gains across R&D, manufacturing, and administrative functions. In a U.S. market shaped by pricing pressure, policy changes, and portfolio crowding, this margin expansion is particularly critical.
Agentic AI is a near-term operating advantage for U.S. pharma. The organizations that succeed will not be those that run isolated pilots, but those that:
In a market where regulatory confidence, speed, and execution excellence define winners, Agentic AI offers U.S. pharmaceutical companies a way to scale trust while moving faster than ever before.
Agentic AI delivers value in ways that traditional automation and standalone GenAI tools cannot. Its advantages lie in how work is owned, governed, and scaled in one of the world’s most regulated industries.
Unlike conventional GenAI tools that generate text in isolation, Agentic AI is designed to operate inside regulatory frameworks. Each action, whether drafting, validating, or updating documentation, is logged, traceable, and aligned with predefined compliance rules.
For U.S. pharma companies working under FDA oversight, this means:
Agentic systems systematize regulatory discipline at scale.
Traditional AI tools improve individual steps in a process. Agentic AI takes responsibility for the entire workflow.
Once given a defined objective, such as preparing a submission package or maintaining post-market documentation, agentic systems coordinate tasks across data sources, applications, and teams. They manage dependencies, monitor progress, and escalate exceptions only when human input is required.
This end-to-end ownership is especially valuable in U.S. pharma, where delays often occur not due to lack of expertise, but due to fragmented handoffs between teams and systems.
U.S. pharmaceutical companies face a persistent shortage of experienced talent in regulatory affairs, medical writing, and quality functions. Agentic AI addresses this challenge by capturing institutional knowledge and embedding it into execution.
By learning from approved submissions, internal SOPs, and expert feedback, agents apply best practices consistently across projects, regardless of team size or geography. This allows organizations to:
In effect, Agentic AI turns scarce expertise into a scalable asset.
.png)
One of Agentic AI’s most distinctive advantages is its ability to handle work that is impractical for human teams, not because it lacks importance, but because it is too complex or continuous.
Agents can:
For U.S. pharma companies operating at scale, these capabilities help surface risks and opportunities earlier, often before they become visible through traditional reporting.
Speed is a competitive advantage in U.S. pharma, but accelerating timelines has historically increased risk. Agentic AI breaks this trade-off.
By embedding validation, governance, and human-in-the-loop checkpoints into execution, agents move work forward faster while maintaining control and transparency. This allows leadership teams to pursue faster development and submission cycles without compromising trust with regulators.
Beyond productivity gains, Agentic AI supports long-term margin resilience. By reducing rework, minimizing delays, and improving asset throughput, agents contribute to both revenue growth and cost efficiency.
As pricing pressures and policy changes continue to impact U.S. pharma, this ability to expand margins through operational excellence becomes increasingly critical.
For U.S. pharmaceutical companies, the true advantage of Agentic AI lies in its ability to redefine how regulated work gets done. It transforms execution from a people-constrained function into a scalable, governed system, one that moves as fast as science allows, without losing regulatory confidence.
At Antino, we help U.S. pharmaceutical companies move beyond pilot programs and build Agentic AI systems that function reliably in regulated, real-world environments. Our teams combine strong engineering capabilities with a clear understanding of FDA-aligned workflows to design solutions that are secure, auditable, and scalable.
From regulatory documentation and clinical operations to quality and compliance processes, we ensure Agentic AI integrates smoothly with existing platforms so innovation accelerates without disrupting ongoing operations.
We also work closely with leadership teams to translate Agentic AI into measurable business impact. This includes identifying high-value workflows, establishing governance and oversight models early, and creating systems that scale expertise rather than headcount.
Whether the objective is faster regulatory submissions, improved operational efficiency, or long-term margin resilience, Antino helps life sciences organizations make Agentic AI a core operating capability that delivers sustained value. Get in touch!

